MnO2 nanorods, Titanate nanotubes
Physical and chemical properties of manganese dioxide, that exist in several polymorphic forms (α, b, g, d, …), originate from structure where basic building blocks, MnO6 octahedra, connect in different ways creating layer and tunnel structures.
Nowadays MnO2 is exploited in various technological applications such as cathode material in lithium ion batteries, sensors, various magnetic applications, in catalysis, and due to their porous structure they are also used as molecular sieves.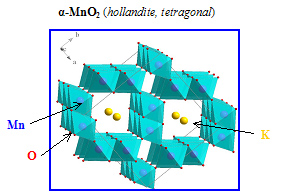
Double chains of MnO6 octahedra form 2 x 2 tunnel structures by edge and corner sharing.
Formed tunnel structures show high selectivity for the intercalation of cations with an effective ion radius of about 1.4 Å (K+, Ba2+, etc.) and also small molecules.
Within tunnel structures, cations balance negative charge in Mn-O network and thus consequently affect physical properties of a-MnO2.
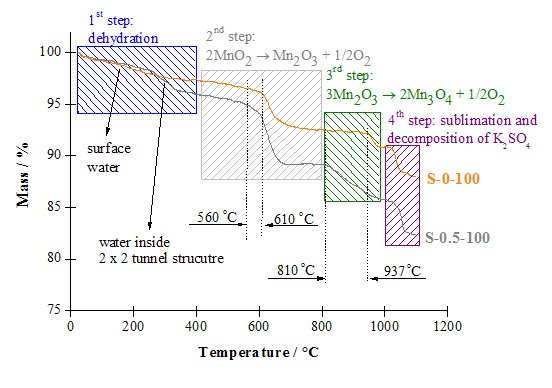
Dynamic TG curves in an inert atmosphere. Label S-0-100 denotes sample with higher K+ content (6.18 wt.%), while S-0.5-100 sample with lower K+ content (2.98 wt.%).
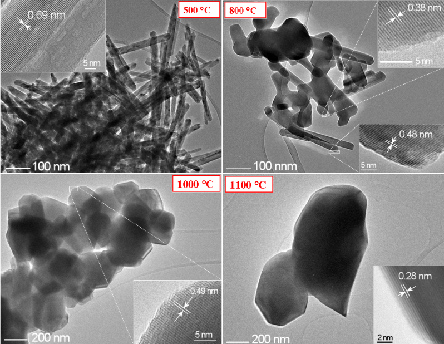
TEM images of the sample S-0-500 and structural changes during thermal treatment.
